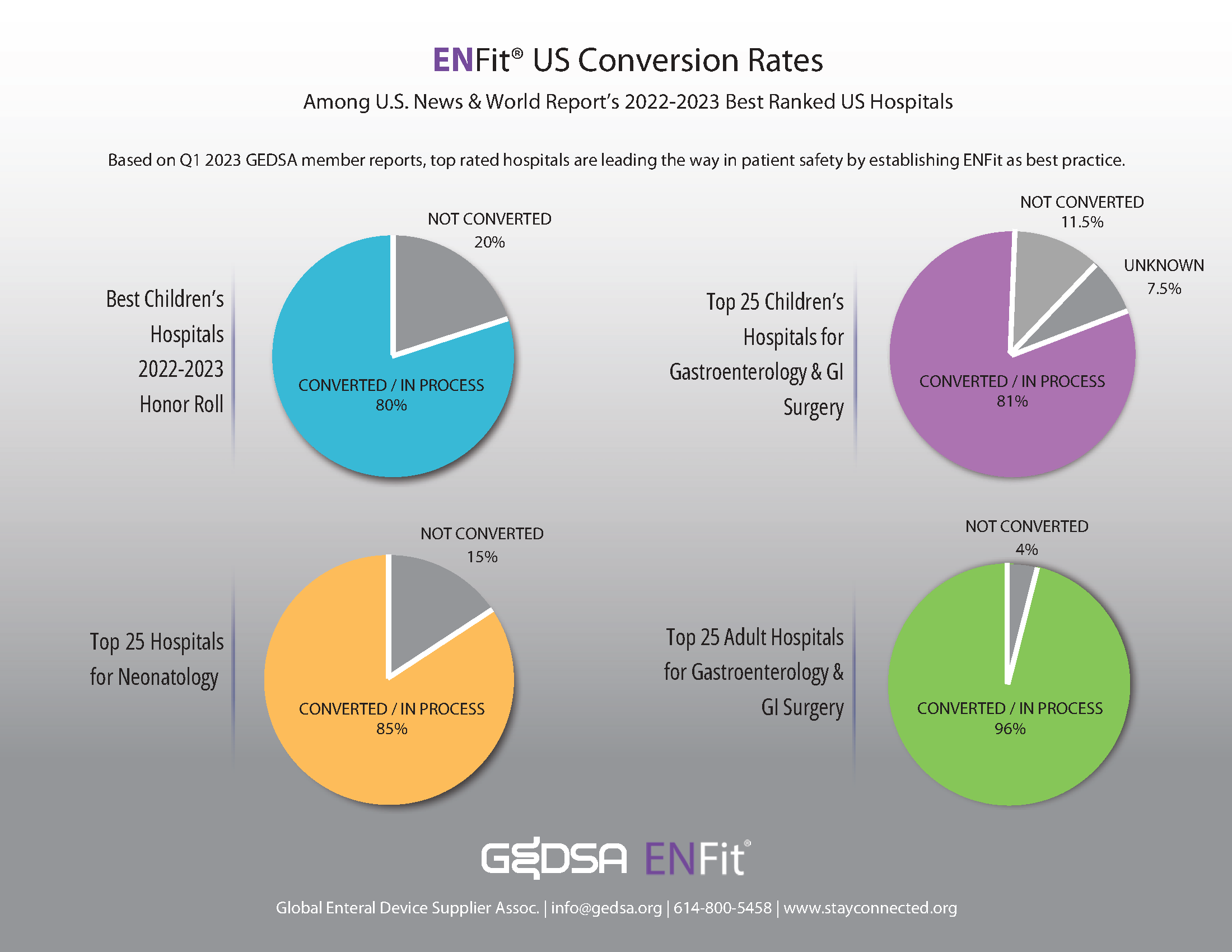
Top ranked US hospitals are leading the way in establishing ENFit as a best practice for patient safety.
News
15 items

GEDSA is a 501(c)(6) federal tax exempt Non-Profit Trade Association incorporated in the State of Ohio, USA. GEDSA’s mission is to promote initiatives surrounding safe and optimal delivery of enteral feeding and connectivity. It is GEDSA’s policy and the responsibility of every GEDSA member to comply in all respects with federal, state and international antitrust laws. Any discussion is strictly prohibited for the purpose of raising, lowering, or stabilizing prices; regulating production; allocating markets; encouraging boycotts; fostering unfair trade practices; or assisting monopolization at any GEDSA function. Any questions regarding the meaning or applicability of this policy, as well as any concerns regarding activities or discussions at GEDSA functions, should be promptly brought to the attention of GEDSA’s executive director, officers, and/or legal counsel.
All provided materials are intended for informational purposes only and should not be used to replace regulatory or company-specific documents, nor should they replace the advice of a qualified professional. This information is based on the ISO 80369 standards; does not imply or suggest any regulatory clearance of any specific product and may not be predictive of clinical outcomes. The images provided do not imply endorsement of any specific product. All products and product designs are the responsibility of each specific legal manufacturer, distributor, or supplier. Products with these design features may be pending regulatory clearance or may not be available in a specific geography. Consult your supplier representative for product-specific availability, indications, contraindications, precautions, and warnings.