Enteral Device Product Discontinuation Update:GEDSA Members and Affiliates Share ENFit® Transition Support & Product Shift Timelines to Increase Patient Safety
Earlier this year, GEDSA manufacturers submitted letters with their manufacturing plans for conversion to ENFit. Those statements were consolidated into one unified document linked here. Recently, Cardinal Health and MOOG submitted updates to confirm that they were adhering to their schedules and are on track as originally communicated.
May 25th, 2021 Dear Valued Customer, On September 14th, 2020, the Global Enteral Device Supplier Association (GEDSA) announced that member manufacturers will phase out legacy enteral feeding products and transition adapters. This transition will meet ISO standard 80369-3, commonly referred to as ENFit®.
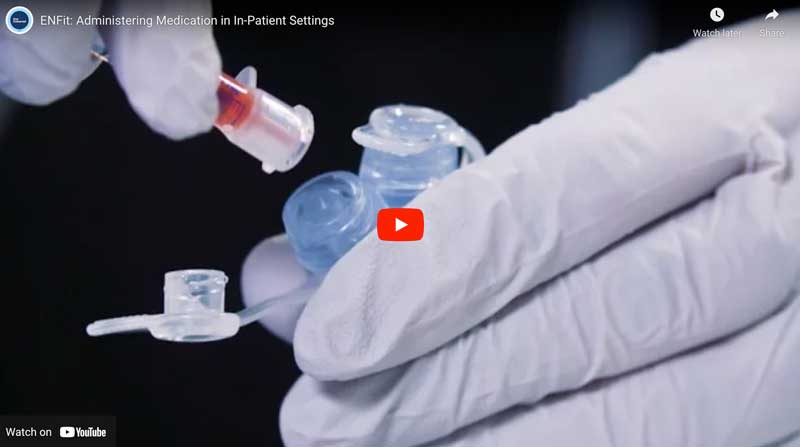
Purpose: Outline the preparation and administration of medication doses using the ENFit system. This procedure will be applicable to patients of all age groups in inpatient settings. Date: October 4th, 2016 | Length 9:28
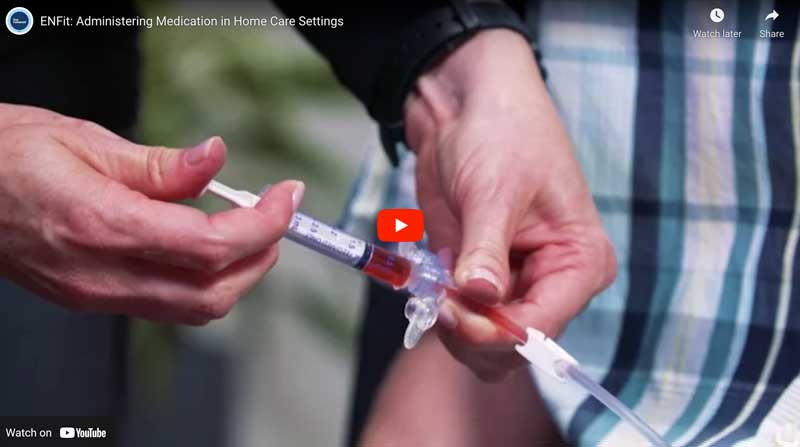
Purpose: Outline the preparation and administration of medication doses using the ENFit system. This procedure will be applicable to patients of all age groups in Home Care settings. Date: October 4th, 2016 | Length 4:32