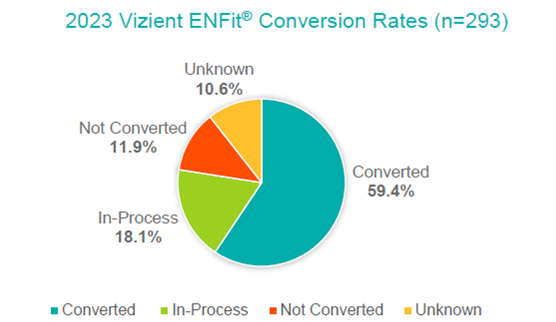
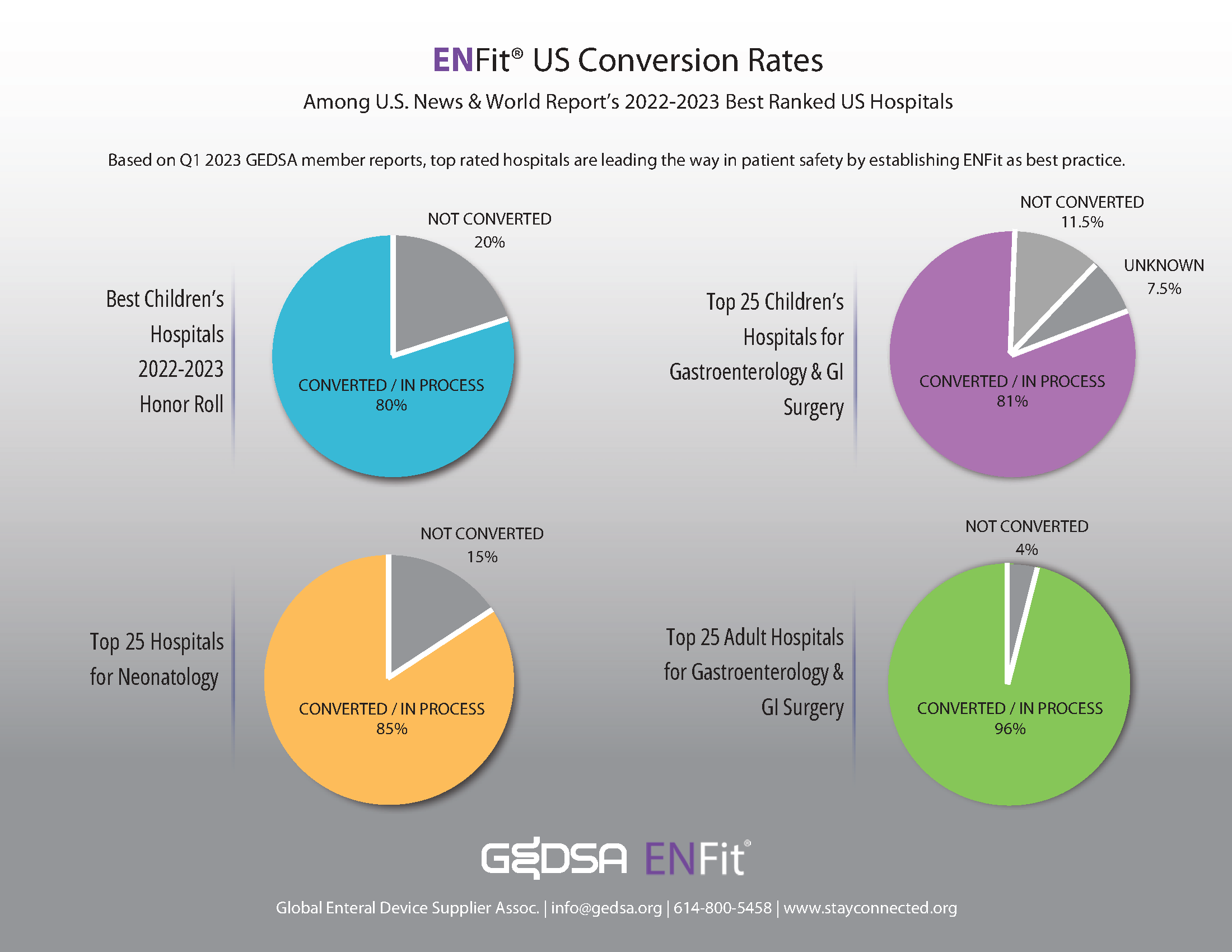
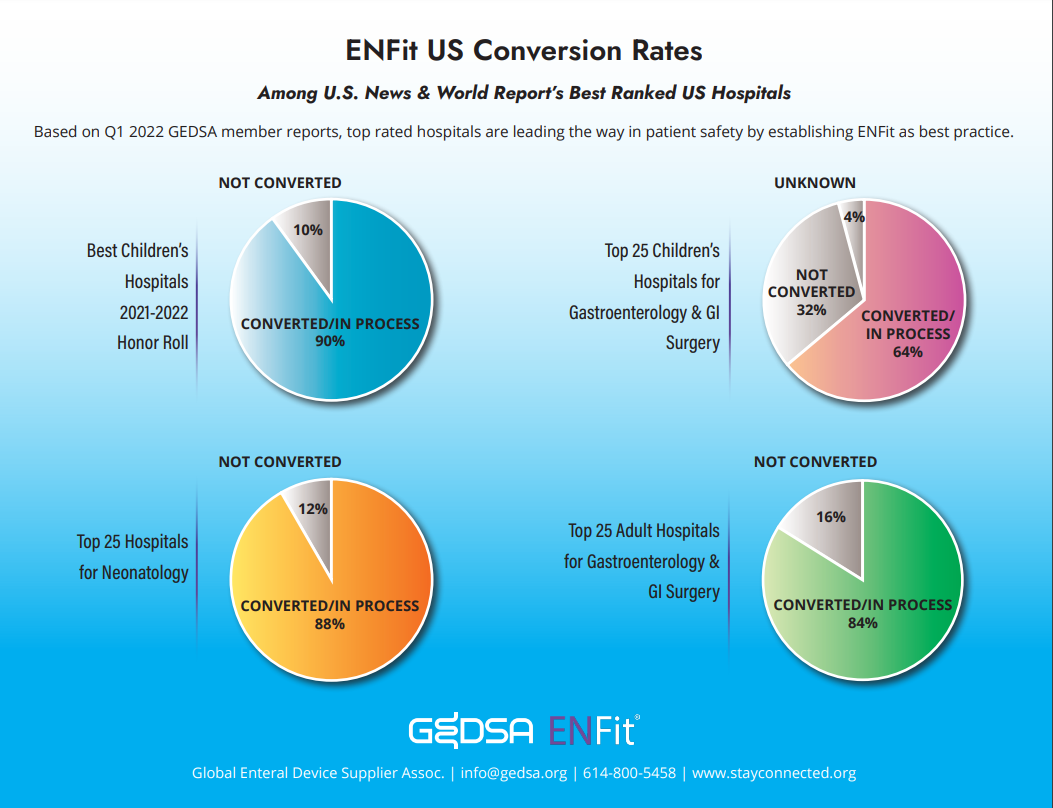
Top ranked US hospitals are leading the way in establishing ENFit as a best practice for patient safety.
June 27, 2022 Item: Conversion to enteral products (e.g., feeding tubes, adapters, syringes) with ENFit® connectors
Any health care setting - whether it be a small clinic, a major surgery center, or a home health care environment - has a plethora of equipment to ensure safety and deliver care. IV’s, catheters, feeding tubes, blood pressure inflation tubes, medical gas supply lines...
On September 14, 2020, the Global Enteral Device Supplier Association (GEDSA) released a revised ENFit® Connector Conversion Schedule for the U.S. and Canada to phase out legacy enteral tubing connectors and switch to ISO 80369-3 compliant ENFit®-only connectors; which improve patient safety by reducing the risks of potentially fatal medical device misconnections and minimize unintentional disconnections, which can lead to the loss of prescribed nutrition.
t is understandable that any transition or change can be daunting. Converting your facility from legacy connectors to ENFit is no exception; however, it is our hope that a deeper understanding of the process will lessen resistance and encourage you to begin. In this article we will address common myths and facts around the process of converting, specifically how demand affects current and future supplies of both ENFit and legacy devices. We will also highlight the importance of communication when it comes to planning a successful conversion.
ENFit US Conversion Rates Among U.S. News & Worlds Report Best Ranked US Hospitals